Cannabis legalization just took another step forward thanks to a Health Canada announcement on Friday.
On June 14th, the agency released a proposal for updated Cannabis Act regulations pertaining to edible cannabis, cannabis extracts and cannabis topicals.
See also:
- Victoria’s newest cannabis dispensary is opening on Gorge Road East this Saturday
- Cheech and Chong’s ‘O Cannabis Tour’ coming to Victoria this fall
These products will be legally available for recreational sale as of October 17, 2019, however suppliers must notify Health Canada 60-days before anything can be sold.
For this reason, no edibles, extracts, or topicals are expected to be on the shelves before mid-December.
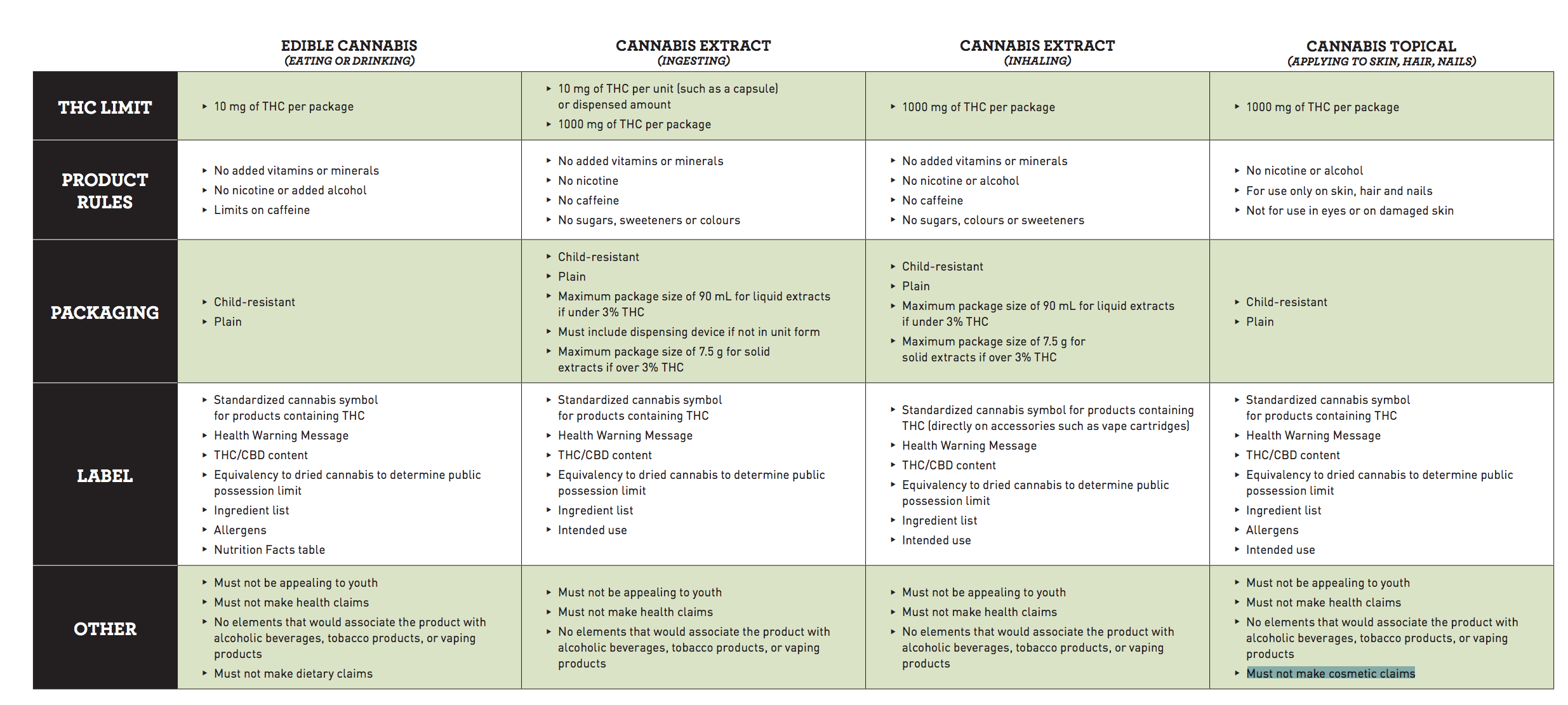
All products will have to adhere to strict branding requirements, cannot appeal to children, bear distinct THC markings, or associate the product with alcoholic beverages, tobacco products, or vaping products.
Here are the specifications for each type of product:
Edibles (eating or drinking)
- 10 mg of THC per package
- No added vitamins or minerals
- Limits on caffeine
- Standardized cannabis symbol for products containing THC on the package
- Health warning message, ingredient list, allergens, nutrition facts on the label
Cannabis extract
- 10 mg of THC per unit (such as a capsule) or dispensed amount, and 1,000 mg of THC per package
- No added vitamins or minerals, nicotine, caffeine, sugars, sweeteners, or colour
- Maximum package size of 90 mL for liquid extracts if under 3% THC
- Maximum package size of 7.5 g for solid extracts if over 3% THC
Cannabis topical (applying to skin, hair, nails)
- 1,000 mg of THC per package
- For use only on skin, hair and nails – not for use in eyes or on damaged skin
- Packaging must not make cosmetic claims